The companion diagnostics development services market, is anticipated to grow at a CAGR of over 10%
Given the various challenges associated with co-development of companion diagnostics and drug / therapy, drug developers prefer to rely on third-party service providers that offer customized services and advanced technologies.
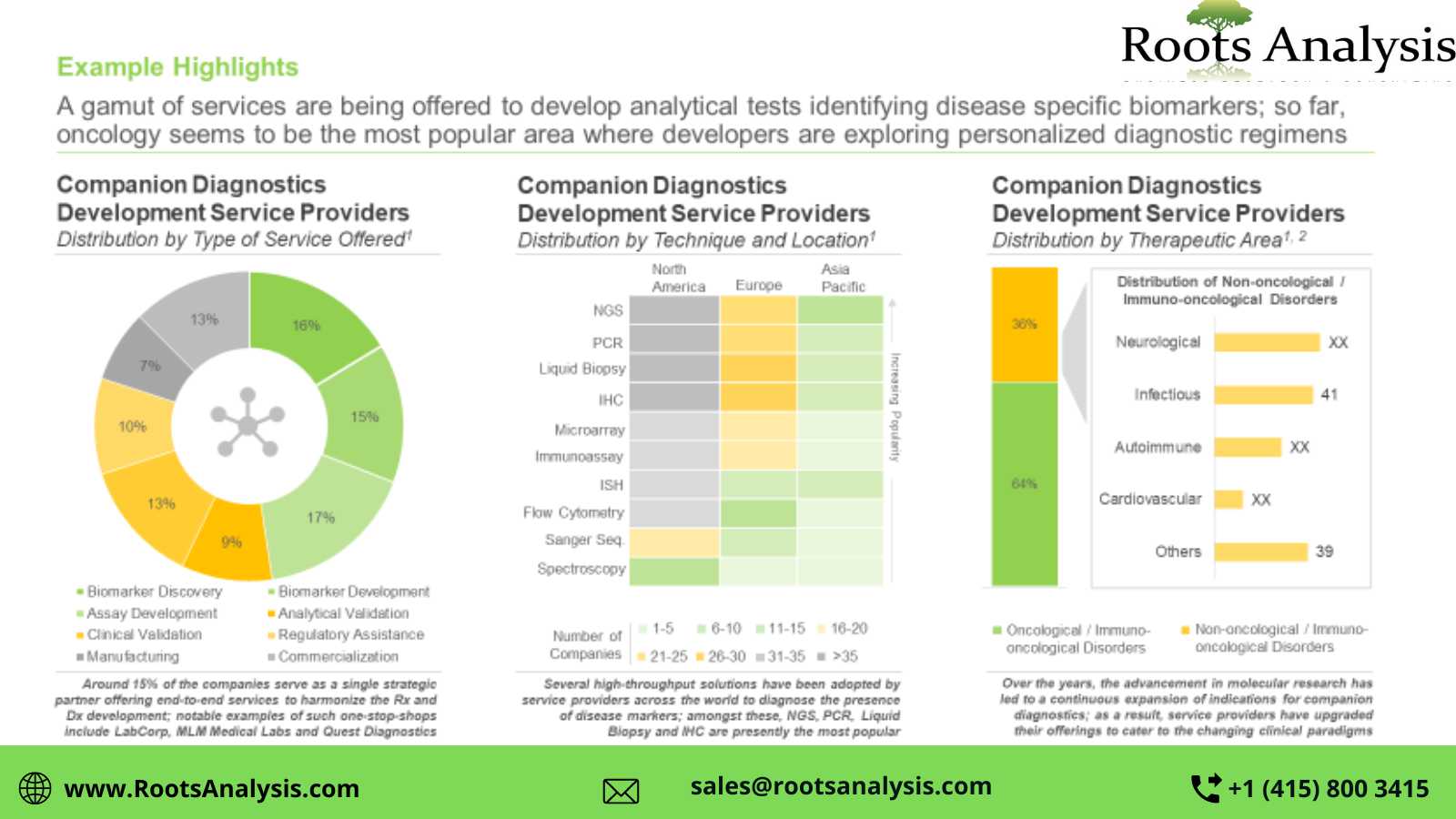
The growing pipeline of patient-centric targeted therapies has led to a surge in demand for companion diagnostics; these tests are known to improve the success rates of late-stage trials by almost three-fold. The development and approval of these FDA classified high-risk devices requires multidisciplinary expertise and an established network of R&D and production facilities that can be accessed through service providers.
For additional details, please visit
https://www.rootsanalysis.com/....reports/view_documen